Systematic Reviews and Literature Reviews
Systematic reviews are considered the gold standard of evidence synthesis. A systematic review does not just mean a literature review that has been conducted systematically. A systematic review is a specific type of research methodology that has its own set of standards and methods. Systematic reviews are typically written with a team and include protocols or rules on how they are conducted. UNCG librarians can help you learn more about systematic reviews, so be sure to contact your liaison librarian for assistance.
UNCG Libraries Systematic Review Guide
This page was modified from the USC Libraries Choosing Evidence tutorial.
Systematic literature reviews are at the top of the levels of evidence pyramid, but other literature reviews are not. Literature reviews, traditional literature reviews, or narrative literature reviews are considered expert or consensus opinion.
| |
Systematic Review |
Literature Review |
| Definition |
Identifies, selects, appraises, and synthesizes all research evidence related to that question. |
Qualitatively summarizes evidence on a topic using informal or subjective methods to collect and interpret studies. |
| Goals |
Answer a focused clinical question
Eliminate bias
Provide a secondary analysis of existing research
|
Provide summary or overview of topic |
| Question |
Clearly defined and answerable clinical question
Recommend using PICO as guide
|
Can be a general topic or a specific question |
| Components |
Pre-specified eligibility criteria
Systematic search strategy
Assessment of the validity of finding
Interpretation and presentation of results
Reference list
All components are tested, turned into a protocol that is shared and finalized before the full review is carried out
|
Introduction
Methods
Discussion
Conclusion
Reference list
May include systematic review components such as eligibility criteria but it is acceptable to adjust these as needed during any part of the project
|
| Number of Authors |
Three or more
|
One or more
|
| Timeline |
Months to years
Average 18 months
|
Weeks to months |
| Requirements |
Thorough knowledge of topic
Perform searches of all relevant databases
Additional searching and browsing to find all published and unpublished research on the question
Use a checklist or similar tool to standardize the appraisal of research studies
Statistical analysis resources (for meta-analysis)
|
Understanding of topic
Perform searches of one or more databases
|
| Value |
Connects practicing clinicians to high quality evidence
Supports evidence-based practice
|
Provides summary of literature on a topic |
Table modified from USC Libraries Choosing Evidence tutorial
For more information about literature reviews: UNCG Libraries Literature Review Module
When searching for systematic reviews, you can usually spot a systematic review by the title.
- The title of a systematic review should include “systematic review,” or “meta-analysis,” or even “systematic review and meta-analysis.”
- Some health science databases, such as PubMed, have a filter that includes “systematic reviews.”
- The title of a literature review will include “literature review” or "narrative review," and sometimes just “review.”
- Keep an eye out for scoping reviews or integrative reviews. These are other literature reviews that find and synthesize evidence, but they are not systematic reviews.
- Keep an eye out for systematic review protocols. These are the blueprint/plan for conducting a systematic review but not the completed systematic review.
If creating a systematic review, keep in mind that systematic reviews are different from literature or narrative reviews. A systematic review addresses a "...clearly formulated question that uses systematic and explicit methods to identify, select and critically appraise relevant research, and to collect and analyze data from the studies that are included in the review." (Cochrane Handbook). Because of this, the time commitment for a systematic review is significant, please plan ahead and be sure to leave enough time to work on your review.
Month Activity
1 – 2 Preparation of protocol.
3 – 8 Searches for published and unpublished studies.
2 – 3 Pilot test of eligibility criteria.
3 – 8 Inclusion assessments.
3 Pilot test of ‘Risk of bias’ assessment.
3 – 10 Validity assessments.
3 Pilot test of data collection.
3 – 10 Data collection.
3 – 10 Data entry.
5 – 11 Follow up of missing information.
8 – 10 Analysis.
1 – 11 Preparation of review report.
12 – Keeping the review up-to-date.
Source: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
From East Carolina University’s Systematic Review LibGuide
A meta-analysis is a statistical analysis of quantitative data sometimes included in systematic reviews that combine studies, such as randomized controlled trials (RCTs), with quantitative and comparable data.
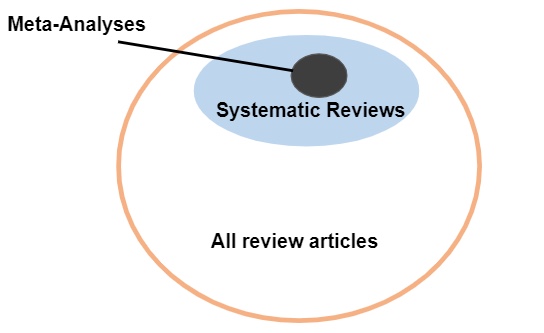
Image from USC Libraries Choosing Evidence tutorial
More guides on systematic reviews:
NIH Systematic Reviews Guide
Quick Check: